DOTXIME
CEFTIZOXIME SODIUM INJECTION
Description
DOTXIME contains CEFTIZOXIME
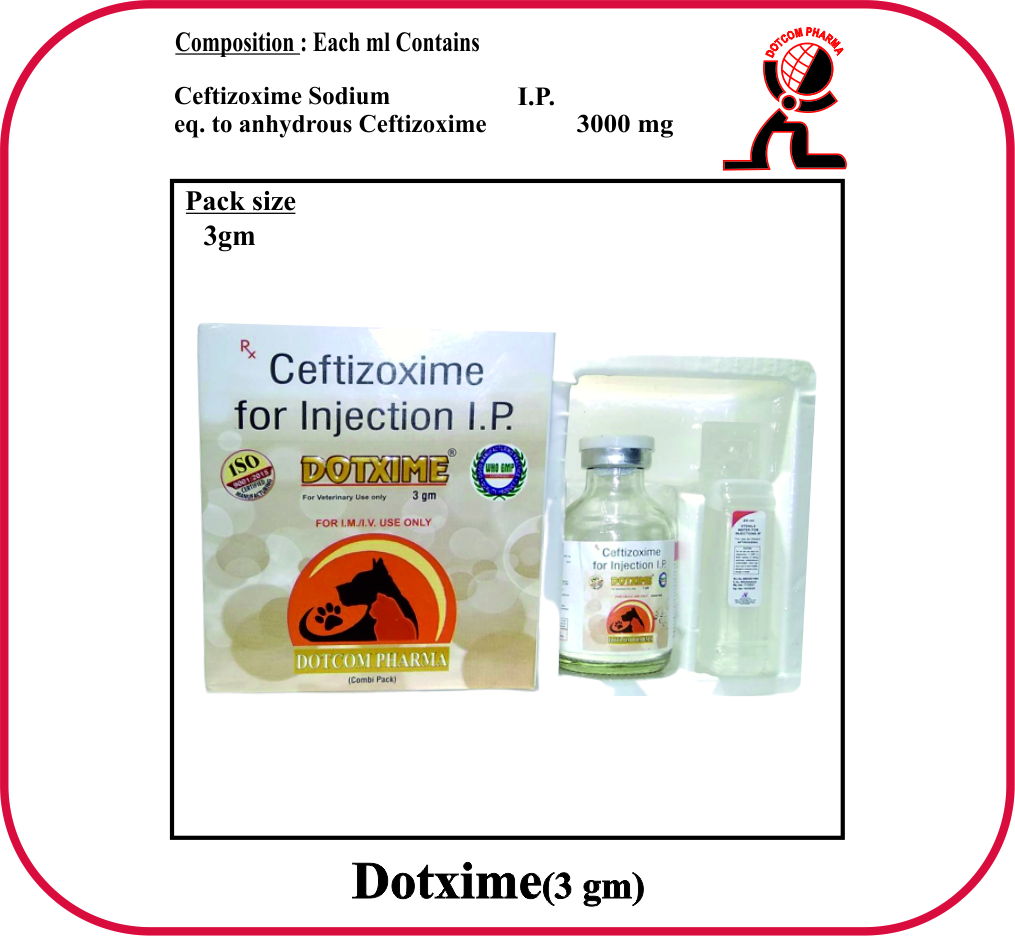
COMPOSITION :-
A) Each vial contains:
Ceftizoxime Sodium U.S.P.
eqv. to ceftizoxime 1.5 gm
B) Each vial contains:
Ceftizoxime Sodium U.S.P.
eqv. to ceftizoxime 3 gm
INDICATION :-
- Clinical Mastitis
- Sub-Clinical Mastitis
DOSE & ROUTE OF ADMINISTRATION :-
By I.V. route after reconstitution at the recommended dose rate,
Cow :
5 mg / Kg Body Weight
Buffalo :
6 mg / Kg Body Weight
Repeat within 96 hrs in severe clinical mastitis cases, if needed.
PRESENTATION :-
1.5 gm, 3 gm
PACKAGING DETAILS :-
| Pack Size | Qty Per Buffer Box | Buffer Box per Case | Qty per Case | Gross weight of Shipper | Dimension of Shipper |
| 1.5 gm | 10 | 15 | 150 | 13.50 Kg | 18.5 x 17 x 12 |
| 3 gm | 10 | 15 | 150 | 13.50 Kg | 18.5 x 17 x 12 |